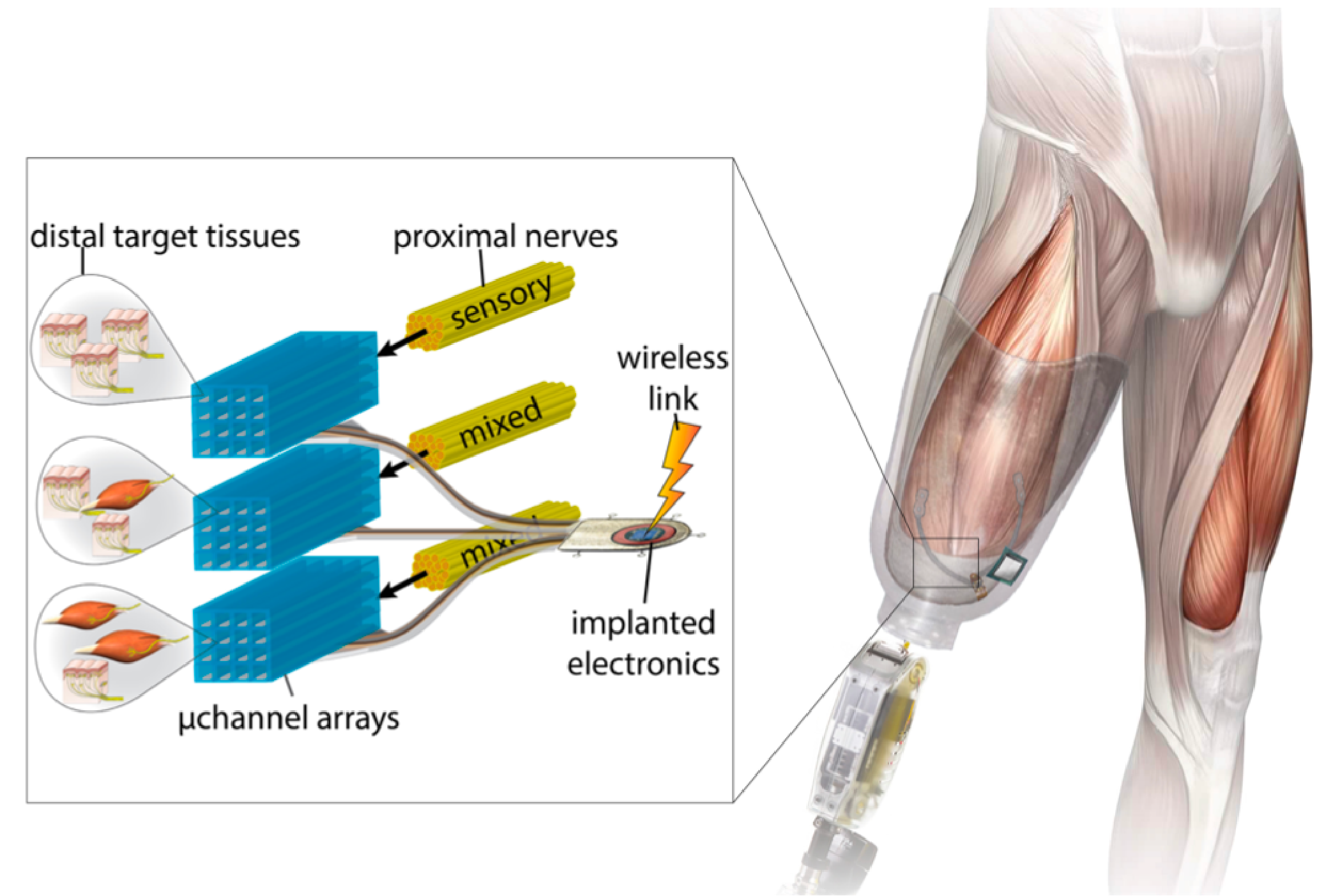
Distal target tissues (skin, muscle) are packed into the residual limb during amputation. Nerves regrow to the target tissues through silicon microchannel arrays, which can selectively stimulate and record from nerve fibers in each channel for closed-loop prosthetic control.
After completing my master’s thesis at MIT on quantum dot LEDs in 2013, I switched labs and worked with Professor Hugh Herr and Dr. Ron Riso in the Biomechatronics Group at MIT Media Lab.
While tremendous progress has been made in the neural control of prosthetic limbs, achieving intuitive, natural feelings of sensation (i.e. not just a buzzing/vibration pattern) and fine motor control remains elusive. This is partially due to the fact that peripheral nerves are mixed bundles of both motor and sensory nerve fibers, making selectively stimulating motor nerve fibers and selectively recording from sensory nerve fibers difficult. I worked on designing and micro-fabricating implantable electronics that we hoped would help tackle this challenge. Specifically, we designed a silicone microchannel array with platinum electrodes that could be implanted during an amputation surgery. As part of the surgery, a mixed motor/sensory nerve in the residual limb would be cut. The array, consisting of hundreds of channels, would be implanted in line with that nerve, whose fibers would regenerate through the microchannels, being forced to separate from one another in the process. Because each channel in our device was individually addressable, the device ideally would allow for selective stimulation of motor nerve fibers and selective recording from sensory nerve fibers, thus enabling much richer and natural schemes of closed-loop prosthetic control. This project was done in collaboration with Draper Laboratory, where I was also a Graduate Fellow.
In conjunction with Dr. Matthew Carty, MD at Brigham and Women’s Hospital, we also wrote a patent describing amputation surgeries that would allow for novel peripheral neural interface schemes. I ultimately left the PhD program at MIT before I finished a PhD to climb mountains, travel, and work at Apple for a few years. Biomech has made tremendous progress in implementing some of these neural interfaces (and more) since my time there, with several of the patent ideas being implemented in practice on real human patients.
related publications
-
Assessment of Nerve Regeneration through a Novel Microchannel Array
Benjamin Maimon, Anthony N. Zorzos, Katherine Song, Rhyse Bendell, Ron Riso, and Hugh Herr
International Journal of Physical Medicine & Rehabilitation, 2016
Advancements in robotic technologies have enabled a significant improvement in the clinical efficacy of prosthetic limbs for persons with upper and lower-extremity amputations. However, significant challenges still remain in establishing a biomimetic bidirectional neural communication between amputees and their external powered prostheses. Regenerative peripheral nerve interfaces may offer a high-resolution alternative to conventional nerve interface technologies for their unique potential to provide increased biospatial resolution for both the control of and feedback from an external powered prosthesis. Here, we present three separate design for 3-D microchannel arrays, each having 16-20 channels measuring 200 μm by 200 μm: one passive (without integrated electrodes), one active (with integrated electrodes), and one active with a porous collagen scaffold. With the array positioned between proximal and distal nerve sendings, we evaluate their effectiveness in tibial n. regeneration in vivo in both rats (N=4) and ferrets (N=4). Using immunofluorescence, we report robust mixed sensory and motor nerve regeneration through the microchannels in all rats, and weak regeneration in 2 of 4 ferrets, suggesting both interspecies regeneration variability and the lack of benefit of axially oriented collagen in improving ferret nerve regeneration through microchannels.
-
Peripheral Neural Interface via Nerve Regeneration to Distal Tissues
Hugh M. Herr, Ronald R. Riso, Katherine W. Song, Richard J. Casler, and Matthew J. Carty
US Patent US 9,474,634 B2, Oct 2016
At least partial function of a human limb is restored by surgically removing at least a portion of an injured or diseased human limb from a surgical site of an individual and transplanting a selected muscle into the remaining biological body of the individual, followed by contacting the transplanted selected muscle, or an associated nerve, with an electrode, to thereby control a device, such as a prosthetic limb, linked to the electrode. Simulating proprioceptive sensory feedback from a device includes mechanically linking at least one pair of agonist and antagonist muscles, wherein a nerve innervates each muscle, and supporting each pair with a support, whereby contraction of the agonist muscle of each pair will cause extension of the paired antagonist muscle. An electrode is implanted in a muscle of each pair and electrically connected to a motor controller of the device, thereby simulating proprioceptive sensory feedback from the device.